Médecins Sans Frontières/Doctors Without Borders (MSF) called on governments and donors on March 22 to speed up access to new, shorter, safer and more effective treatments* for drug-resistant tuberculosis (DR-TB), as well as the diagnostic tests needed to implement the new treatment regimens. MSF joined the World Health Organization (WHO) and other actors in a ‘Call to Action’ ahead of World TB Day. MSF also urged the US-based diagnostics corporation Cepheid to drop the price of the critical GeneXpert tests to ensure that people with DR-TB can be diagnosed in time to access the shorter and safer treatment regimens.
The WHO issued new guidelines in December 2022 that recommend countries roll out the safer and shorter BPaLM* regimen to treat people with DR-TB, partly based on the results of MSF’s TB PRACTECAL trial, a multi-country, randomised, controlled clinical trial showing that the new, all-oral six-month BPaLM based treatment is safer and more effective at treating DR-TB than the currently used treatment regimens which are longer, cause intolerable side effects and only cure 60% of the people with DR-TB.
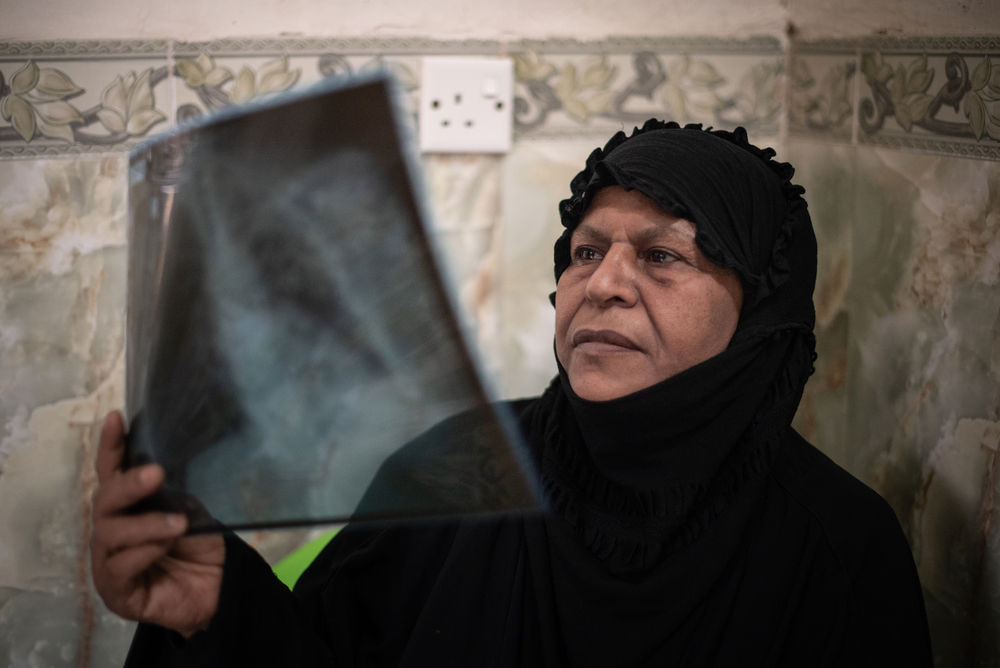
“For me, everything over the past two years has revolved around TB,” said Maria** who underwent two long years of treatment which failed to cure her of DR-TB, and later received the BPaLM regimen in Belarus. “Living like that was impossible. People (on the old treatment) can’t do anything because they feel awful during the treatment. If only I had started (new) treatment with a short course right away, my life would have been different.”
Access to diagnostic testing for resistance is one of the main hurdles to enable rolling out the safer and shorter DR-TB treatment regimens. Currently, the GeneXpert MTB/RIF test made by US corporation Cepheid is the most widely available rapid molecular diagnostic test in high burden countries to detect resistance to the first-line drug rifampicin. Despite high sales volumes in high TB burden countries and MSF’s analysis showing that it costs Cepheid less than $5 to produce one test, the corporation has kept the price of the test at US$9.98 for over a decade now.
Countries should start rolling out the 6-month BPaLM regimen to treat DR-TB, and ensure nation-wide availability of the GeneXpert MTB/RIF tests, or where possible WHO-recommended alternatives such as the Truenat MTB/RIF tests, to detect TB and rifampicin resistance so that people with DR-TB can receive this treatment without any delay.
“It is critical that we have better access to tests to diagnose TB and resistance to drugs used for treating TB so that we can identify more people who need treatment and rollout the shorter and safer all-oral treatment regimens,” said Stijn Deborggraeve, Diagnostics Advisor at MSF’s Access Campaign. “We yet again call on Cepheid to reduce the price of the TB tests to no more than $5 each, so that more people with drug-resistant TB can be diagnosed in time and be offered improved, lifesaving treatments.”
Drug prices also need to come down further: what will help is national TB treatment programmes rolling out these regimens to more people in order to increase demand, as well as having more manufacturers supplying affordable generic versions of bedaquiline and pretomanid. The lowest-available price for this newer DR-TB treatment regimen is still $570, and MSF has called for the price of a complete DR-TB course, including the BPaLM regimen, to be no more than $500. Five countries where MSF works have started implementing the shorter regimens to date include Belarus, Uzbekistan, Tajikistan, Sierra Leone and Pakistan. Further price reductions will pave the way for the rollout of this treatment in many more countries.
“In a country like Afghanistan, where people are struggling to afford basic food items, travel expenses and medical fees for hospital, being able to treat people with drug-resistant TB within 6 months instead of up to two years with the older treatment regimens would be a blessing,” said Dr Geke Huisman, MSF Medical Coordinator in Afghanistan. “Access to affordable diagnostic tests remains a major challenge in Afghanistan and other countries in this region because of the high prices of tests. Governments, donors and pharmaceutical corporations must act now to ensure an affordable supply of these critical tests and treatments for TB, so that more lives can be saved.”
*The six-month BPaLM regimen is composed of bedaquiline (B), pretomanid (Pa), linezolid (L) and moxifloxacin (M). This regimen will not be appropriate for people with TB resistant to bedaquiline, linezolid, pretomanid or delamanid. In parallel with implementing the BPaLM regimen, all countries should urgently scale up access to drug susceptibility testing for the drugs used to treat DR-TB.
** Full name not being shared to maintain anonymity